this is the latest paper from our group, in collaboration with the group of Athanasios Philippopoulos in the University of Athens.
Abstract
The antiplatelet and antithrombotic activity of a novel organometallic rhodium(I) complex of the formula [Rh(cod)Cl(tpc)] (1) (cod = cis‐1,5‐cyclooctadiene; tpc = methyl 2‐amino‐4‐(diethylamino)‐thieno‐[2,3‐d]‐pyrimidine‐6‐carboxylate) was investigated. Complex 1 was easily synthesized by a one‐pot, high‐yield reaction and was fully characterized by standard spectroscopic techniques including FT‐IR, UV–Vis, and NMR spectroscopy and by elemental analysis. The molecular structures of tpc and 1 were determined by single‐crystal X‐ray crystallography. Complex 1 displayed a slightly distorted square planar geometry and is the first crystallographically characterized example of a coordination compound bearing the ligand precursor tpc. Biological studies demonstrate that 1 displays strong antiplatelet and antithrombotic properties in vitro, by inhibiting both the aggregation of human and washed rabbit platelets induced by the potent inflammatory and thrombotic mediator, platelet‐activating factor (PAF) in the micromolar range. This is an approach of continuous interest in the field. Molecular docking calculations suggest that 1 can fit at the ligand‐binding site of the PAF receptor (PAFR) and thus block PAF thrombotic activities, through an antagonistic effect on the PAF/PAFR‐related pathway, which is in accord with the experimental findings. Complex 1 constitutes an interesting example of a metal‐based PAF inhibitor with promising antiplatelet, antithrombotic, and anti‐inflammatory activity, because PAF is the most potent inflammatory lipid mediator. This is also supported by the fact that 1 is an inhibitor of other inflammatory and thrombotic mediators like thrombin, along with well‐established platelet agonists like ADP and collagen.
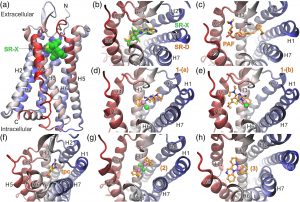
(a) Ribbon representation of PAFR in complex with the antagonist SR27417 from the X‐ray structure with PDB ID: 5ZKP.[38] The antagonist bound within the PAF‐binding site is shown with green C spheres, whereas the seven helices and the N‐ and C‐termini of the receptor are indicated. (b) Close‐up view of the PAF‐binding site from the extracellular (top) side of PAFR illustrating the docked pose of SR27417 (SR‐D) in comparison with the crystallographic structure (SR‐X). The antagonist is shown with green C sticks (X‐ray) or orange C (docked), whereas N atoms are colored blue, O red, and S yellow. (c) Top‐ranked conformation of PAF bound to PAFR, with the P atom colored brown. (d, e) The two most populated clusters of docked poses for complex 1 (1‐(a) and 1‐(b)). Rhodium(I) is shown as a light mauve sphere, the coordinated chloride as a green sphere, whereas cod and tpc ligands are color coded as SR‐D and PAF. Selected, top‐ranked conformations of the bound ligand tpc (f), the precursor complex [Rh(cod)Cl]2 (designated as 2 in g), and the cationic square planar analog [Rh(cod)(pqx)]+ (designated as 3 in h)
Results
Our docking calculations suggest that complex 1 can be accommodated within the PAF‐binding site in two distinct binding conformations (Figure 6d,e), which displayed a marginal difference in their estimated ΔGbinding (Table 5). Interestingly, both the top‐ranked conformation designated as 1‐(a) and the second‐ranked pose 1‐(b) are stabilized via a hydrogen bond between the main‐chain CO of Phe174 and the NH2 group of the tpc ligand (Figure S10). However, in 1‐(a), tpc displays major aromatic–aromatic interactions with Phe174, His176, Tyr177, and Phe152, whereas in 1‐(b), tpc interacts mainly with Trp73, Tyr22, Tyr76, and Tyr77. The cyclooctadiene moiety in 1‐(a) displays hydrophobic–aromatic interactions with Phe97, whereas in 1‐(b) with Phe97 and Phe98 (Figure S10). In comparison with complex 1, the docked conformation of the free ligand tpc exhibits a significantly different pose with the potential for hydrogen‐bonding interactions with Gln252 and His248 (Figures 6f and S10). However, its binding affinity for PAF was estimated to be lower by 2.5 kcal mol−1 (Table 5), which can be attributed to the increased estimated intermolecular energy of 1, albeit with lower electrostatic energy (Table S1). Although a direct comparison cannot be made, the predicted affinity of tpc and 1 is in quite good agreement with the in vitro results obtained from WRPs (Table 5).
Conclusions
In summary, the synthesis and spectroscopic characterization of the rhodium(I) complex 1 incorporating a substituted thienopyrimidine ligand (tpc) was reported. The solid‐state structures of tpc and 1 were determined, for the first time, by single‐crystal X‐ray diffraction. The biological evaluation of the new compounds (tpc and 1) towards the PAF‐induced aggregation in both WRPs and human platelets (hPRPs) showed that complex 1 proved to be a very potent antiplatelet and antithrombotic agent, showing better efficiency than that of the free ligand. The potency of the organometallic compound 1 to inhibit the induced aggregation of human platelets by other well‐established thrombotic mediators and platelet agonists, such as thrombin, ADP, and collagen, was also investigated for the first time. A higher specificity for collagen and PAF is observed, implying that 1 may affect more profoundly the collagen and PAF‐related signaling pathways. In such conditions, where plasma is present, it was found that 1 retained the biological activity in a dose‐dependent way, in contrast to that of organic ligand tpc whose bioactivity was significantly reduced. Molecular docking calculations further suggest that 1 can be accommodated within the ligand‐binding site of PAF receptor and thus block the activity of PAF as a competitive antagonist, which is in accordance with our experimental findings.
Such an overall outcome further supports the possible use of the rhodium(I) organometallic complex 1 as an interesting example of a metal‐based PAF inhibitor with promising, antiplatelet, antithrombotic, and anti‐inflammatory activity against thrombo‐inflammation‐related disorders, tumor metastasis and atherosclerosis, and cardiovascular diseases (CVDs). For the onset and development of these disorders, the potent inflammatory lipid mediator PAF and platelet activation/aggregation are implicated. The results obtained from this study will potentially help us to rationally design new metal‐based compounds with an improved biological profile, which could be used as anti‐inflammatory agents against these disorders in the future.